FRAP (Fluorescence Recovery After Photobleaching) imaging is available as a standalone Benchtop imager or can be added to a Rock Imager 1000. FRAP measures membrane protein mobility in lipids. High protein mobility and fast diffusion rates correlate well with crystallization conditions. The system utilizes an automated high throughput LCP-FRAP assay to prescreen membrane protein crystallization conditions.
FRAP was developed in collaboration with Vadim Cherezov (TSRI) and the JCIMPT center led by Ray Stevens and supported by the NIH Common Fund in Structural Biology.
Identify protein mobility/diffusion in lipids (LCP).
Mobile fraction measurement of 96 wells in less than 45 minutes, 2 plate capacity.
Experiment design and result viewing can all be done with the Rock Maker software.
Compound zoom optics of 1.1 x and 5.5 x objectives.
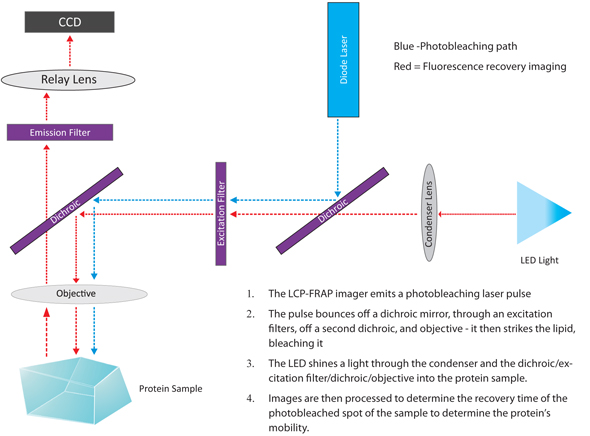
The LCP-FRAP imager bleaches the dye-labeled protein in LCP with a laser pulse. Then, a sequence of post-bleaching images is recorded. The image processing function in the imager software measures the pixel intensity inside the bleaching spot. Protein mobility parameters are extracted from fitting the pixel intensity recovery curve with either single component or double component 2-D diffusion equation.
The LCP-FRAP imager is fully automated to conduct imaging and data analysis for LCP plates.
The diagram below depicts the light path involved in the FRAP imaging method.

The diagram of FRAP imaging method

|
|
| RIC-V30R016 |